Under various medical device regulatory jurisdictions worldwide, the Unique Device Identification (UDI) System is being developed to facilitate adequate medical device identification through distribution and use. Healthcare providers, distributors, group purchasing organizations, payors, researchers, and others are using UDI for supply chain efficiency, tracking medical devices in the electronic health record including track and trace, comparative effectiveness research, electronic procurement, and a multitude of other information. This article, the first in a 2-part series, will give you a quick overview of the basics of UDI and its importance in the track and trace process at medical device companies. In this part, let’s talk through the basics–including what it is and why it matters.
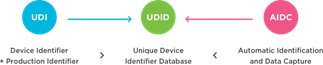
Components of a UDI system: A basic UDI system comprises two numeric or alphanumeric identifiers—Device Identifier (UDI-DI) & Production Identifier (UDI-PI).
-
A Device Identifier (DI) is a mandatory, fixed portion of a UDI system that identifies a manufacturer’s specific product/package configuration, and is used as the "access key" to information stored in a UDI database. Different UDI issuing entities have their specific configurations of device identifiers (i.e., UDI-DI can include the GS1 GTIN or Global Trade Item Number, HIBC-UPN or the Universal Product Number, or ICCBBA ISBT 128-PPIC or Processor Product Identification Code).
-
A Production Identifier is a code that identifies the unit of device production and includes Lot or Batch number, Serial Number, Expiration Date, Date of Manufacture, and/or Version for Software of a Medical Device (SaMD), etc.
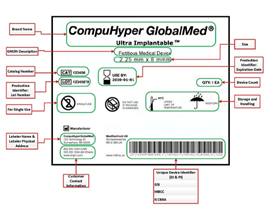
Benefits of a UDI system on medical devices: The UDI system offers a range of benefits to the industry, including regulators, consumers, healthcare providers, and healthcare systems by enabling:
-
Faster discovery of flawed medical devices
-
Faster product recalls of flawed medical devices
-
Reduction in medical errors by healthcare professionals
-
Reduction in counterfeiting of devices
-
More informed patient treatment
-
Better assessment of device performance
-
Improved inventory management
-
More time spent by doctors with patients
-
A world-wide medical device identification system
I bet you are curious to learn more about the mechanics and impact of UDI. Stay tuned for a second part that will attempt to unpack more along these lines.
Image source: The U.S. Food and Drug Administration (FDA)