Emerging Therapeutics: The Cell Therapy Boom
The pandemic acted as a global stress test for the biopharma industry. Governments, private investors, and pharmaceutical companies invested heavily in vaccine research, manufacturing capacity, cold-chain logistics, and platform technologies. mRNA, in particular, proved that complex biologics could be developed, scaled, and delivered on accelerated timelines. This momentum carried forward, fueling a wave of innovation that extends beyond infectious disease. Today’s pipeline reflects a biopharma sector that’s more capable, agile, and equipped to tackle complex therapeutic modalities, many of which were not developed even a decade ago. Biopharma pipelines are increasingly dominated by advanced therapies targeting disease at the cellular, genetic, and molecular levels. Below is a breakdown of the most influential categories shaping the industry’s future in cell therapy.
Cell Therapies
Cell therapies use living cells to repair, replace, or enhance biological functions. Although the foundations for many of these treatments have existed for years, their emergence into the commercial pipeline is the direct result of recent advancements in bioprocessing, automation, analytics, and cold-chain transport. These treatments are transforming care for cancer, rare diseases, and immune disorders. However, their complexity requires extraordinary precision across manufacturing, storage, and packaging.
Why Cell Therapies Are Emerging Now
- Manufacturing technologies have matured, enabling more consistent, scalable cell expansion.
- Cryogenic logistics infrastructure expanded significantly during COVID, making deep-cold transport more attainable.
- Regulatory bodies now have clearer frameworks for cell-based products, accelerating timelines.
- Advancements with digital systems that are used to link patients to their individual biological materials or therapies (known as chain-of-identity, or COI tracking) have helped to make autologous therapies more feasible on a commercial scale.
Emerging Cell Therapies driven by Source
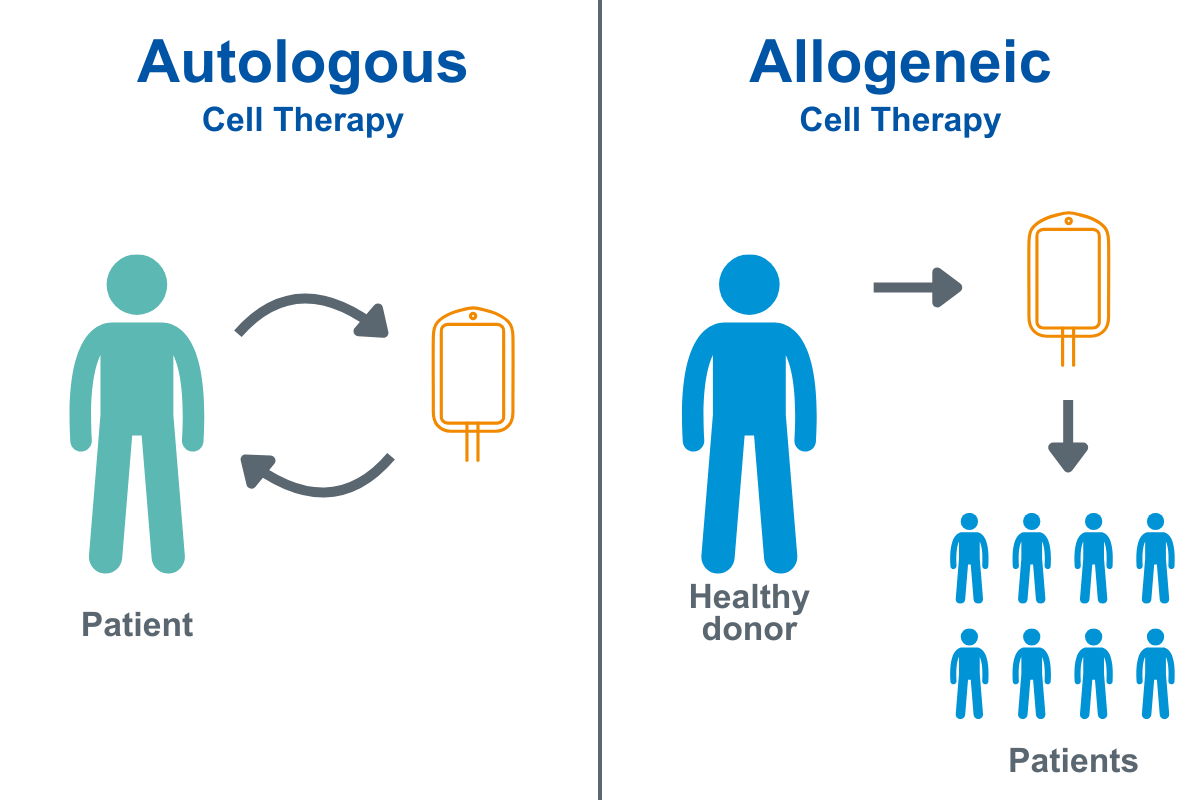
Autologous Therapies
Cells are collected from the patient, modified or expanded in controlled environments, and reinfused back into the same patient. These are highly personalized therapies requiring strict COI tracking, specialized packaging, and precise logistics. Autologous therapies are emerging more rapidly today because biopharma has digital, logistical, and manufacturing systems to support individualized batches, something that was not feasible on a large scale a decade ago.
Allogeneic Therapies
Cells are sourced from a donor and used as an “off-the-shelf” treatment for multiple patients. Allogeneic programs aim for greater scalability but require robust bioprocessing, standardized packaging formats, and long-term storage capabilities. Allogeneic therapies are emerging now due to advances in cell engineering, immunotherapies, and quality control that help reduce rejection risks and scale up manufacturing.
Emerging Cell Therapy Types Driven by Disease
There are several illnesses that have prompted specialized engineering and programming of cells to combat/fight the illness. Hematopoietic Stem Cell Transplantation (HSCT) is one of the earliest and most established cell therapies. HSCT uses blood-forming stem cells to treat blood cancers and disorders. HSCT is not a new therapy, but serves as the foundation for several more advanced cell-therapies that have been developed, which include:
- CAR T-cell therapy: This involves genetically altering patient cells (i.e., gene modification) to enhance disease-fighting function. T-cells are engineered with a Chimeric Antigen Receptor (CAR) to target cancer cells with high precision. CAR-T has rapidly expanded due to advances in gene editing tools, closed-system cell processing, and improved cryopreservation technology.
- Tumor-Infiltrating Lymphocyte (TIL) therapy: This involves genetically altering patient cells and occurs when lymphocytes are extracted from tumors, expanded, and then reinfused to attack solid tumors. Once limited to research centers, TIL therapy is becoming widespread as modern automation and analytics now support consistent cell expansion at scale.
- Mesenchymal Stromal Cell (MSC) Therapy: The therapy uses mesenchymal stem cells to suppress immune response or promote tissue regeneration across inflammatory and degenerative conditions. MSC therapies are emerging thanks to better characterization methods, improved potency assays, and modern closed-system bioreactors.
- Dendritic Cell Vaccines: These cells are trained to recognize tumor antigens, boosting the immune system’s ability to detect and destroy cancer cells. Recent advances in tumor antigen discovery, high-throughput sequencing, and closed-system cell-processing technologies have brought this once-experimental approach into a new phase of clinical development.
The post-COVID surge in investment, infrastructure, and scientific capability has influenced what is possible in biopharma. Cell therapies that once lived only in research settings are now advancing toward commercial availability, and supported by strong manufacturing systems, expanded cold-chain capacity, and clearer regulatory pathways. As the industry continues to shift toward therapies that operate at the cellular and genetic level, biopharma companies and their industry partners strive to innovate across every stage of development, from early research to packaging, storage, and delivery. The momentum generated over the past five years has positioned the industry for a future where highly personalized, highly potent treatments are not the exception but the expectation.
Sources
- https://www.cap.org/member-resources/articles/allogeneic-cellular-therapy-diagnostic-challenges-and-opportunities-for-laboratory-practice https://www.sciencedirect.com/science/article/pii/S2666634025001047
- https://blog.dana-farber.org/insight/2017/03/autologous-vs-allogenic-stem-cell-transplants-whats-the-difference/
- https://patienteducation.asgct.org/understanding-cell-gene-therapy/what-is-cell-therapy



