The In Vitro Diagnostics (IVD) industry is fascinating, and as precision medicine is prioritized more heavily around the world, it is important that we understand the industry from even the most basic level. When I was talking with a colleague recently about packaging for the IVD industry, it became clear just how diverse the landscape is, and how quickly it is evolving. For example, specialized DNA testing, a wearable glucose monitor, and a simple urine test are all considered diagnostics. To put it simply, anything that is used to provide a diagnosis belongs in this category. Additionally, as I’m sure you can imagine, the setting for package opening and use are quite different too, depending on the IVD. For example, a diagnostic might be used in a regulated setting like a healthcare facility, hospital, or a laboratory. Some diagnostics though, like allergy testing kits, are used in personal homes.
Fig. 1 On the left, we see how air can flow into and through a Porous material. On the right, we see a Non-Porous material.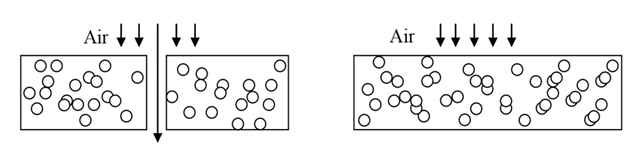
As I said, the IVD industry is evolving so quickly that understanding how sensitive a diagnostic is to moisture or oxygen, for example, is a lot like playing a guessing game. Product engineers must know how sensitive their product is to moisture, light, gas, and more. In many cases, if the sensitivity level is unknown, packaging professionals default to the worst case scenario. That’s why utilizing a conservative material like foil is so common. You want to control what goes into and comes out of your package as much as possible, and because foils are so densely packed, there is less space—at the molecular level—for water, oxygen, or gases to come in.
Fig. 2 Note how densely packed foil molecules are in the left image, making the foil very difficult to penetrate. On the right, we included a barrier film for comparison.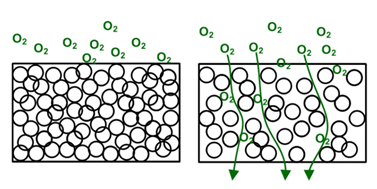 Here are a few examples from the medical device industry to help demonstrate product sensitivity, and why it is so important to control the package atmosphere:
Here are a few examples from the medical device industry to help demonstrate product sensitivity, and why it is so important to control the package atmosphere:
-
For something like dissolvable sutures, which are meant to dissolve in your body, it is crucial that the package contents stay dry to prevent degradation prior to use. In this instance, you would need to keep water out. In other words, you’d want to keep the outside atmosphere—which we know is made up of moisture, oxygen, and more—from coming into the package. Otherwise, the sutures may degrade (or even break) prior to or during the point of use.
-
For hydrogel packaging, on the other hand, you must maintain moisture without escape to prevent the gel from drying out prior to use.
Similarly, in the IVD industry, reagents can be impacted by gas, moisture, and more, which could render the diagnostic test outcome invalid. That’s why when packaging any diagnostic, taking a conservative approach is common. With a better understanding of product sensitivity, GTR, and material science, I would argue that packaging professionals gain some flexibility and are able to utilize far more than just foil for their packaging.
IMAGE SOURCE: Modified Atmosphere Packaging


.png)

 Here are a few examples from the medical device industry to help demonstrate product sensitivity, and why it is so important to control the package atmosphere:
Here are a few examples from the medical device industry to help demonstrate product sensitivity, and why it is so important to control the package atmosphere:
