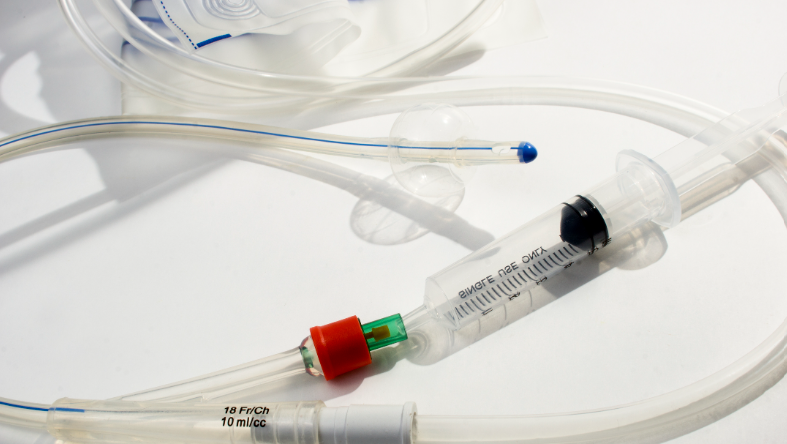
Oliver’s Award-Winning Dual Hoop Catheter DISK
Our Dual Hoop Catheter DISK continues to be on the cutting edge of medical device packaging design and was honored most recently as the winner of Packaging Design of the Year at the Healthcare Asia Medtech Awards 2021.
Because of its innovative design, catheter wires and procedural components are housed in one convenient system, eliminating the need to manufacture extra pouches, shelf cartons, and corrugated shipping boxes for each individual component. Packaging waste is reduced by 20-30% and significant cost and time savings are realized by medical device manufacturers.