How to Measure and Select the Best Medical Packaging Materials
When facing customer inquiries about packaging materials, some of the most common questions we encounter are: What is the thickness of the film material; how do you convert mil and ga; and, how thick is X ga material? When selecting packaging materials, material thickness is a very intuitive concept. Due to differences among regions, industries, material characteristics, and more, different materials use different units of measurement for description.
A proficient understanding of the relationship between units of measurement, combined with material characteristics, composition, and other aspects, provides better direction when choosing packaging materials. This is especially true in the field of medical packaging, where the final selection of material specifications directly relates to the quality of medical products, the safety of medical instruments, and the well-being of patients.

Let’s explore a few common packaging materials.
Tyvek®
Tyvek® materials produced through a flash vaporization process for medical use exhibit variations in thickness and cannot be labeled solely by thickness. Instead, they are categorized based on the weight of material per square meter. In terms of touch, the three types of Tyvek exhibit slight differences in thickness.
Tyvek® 1073B - Approximately 74.7 g/m² - Provides the highest level of protection in terms of strength and microbial barrier.
Tyvek® 1059B - Approximately 64.5 g/m² - Offers reliable sterile protection for medium-risk medical instruments.
Tyvek® 2FS - Approximately 59.5 g/m² - Features a durable puncture-resistant substrate that surpasses paper in terms of resilience and microbial barrier.
Tyvek® materials are lightweight, thin, and strong, possessing excellent properties such as high breathability, strong microbial barrier, puncture resistance, and low dust generation. They are particularly suitable for packaging Class II and Class III medical devices.
Film
Film materials are typically produced through extrusion processes, resulting in relatively uniform thickness. Commonly used units include mil, ga, μm (micrometers), 丝(S)and mm (millimeters). Among them, mil and ga are imperial units, while μm and mm are metric units. Additionally, 丝(S)is not a standard or legally recognized metric unit but an ancient Chinese measurement unit that has been retained and is still commonly used due to industry convention and measurement convenience.
Unit Conversions:
1 ga = 0.01 mil
1 mil = 100 ga = 25.4 μm = 0.0254 mm
1 丝(S)= 10 μm = 0.01 mm
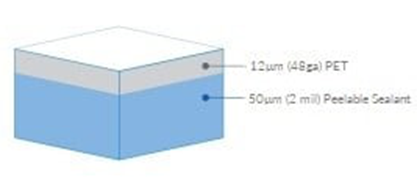
Medical film materials are usually composed of multiple layers, including the surface layer, barrier layer (optional), and sealing layer. The total thickness of the (raw) material after composition typically ranges from 50 to 150 μm, determined by the materials used.
 |
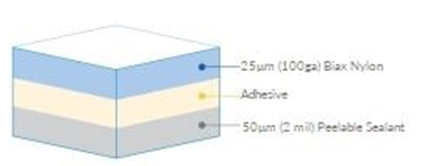 |
Furthermore, to meet the specific requirements and applications of medical devices, different layers of materials offer various choices to address diverse protective and packaging needs. For instance, materials include those with high puncture resistance like nylon (PA) and materials with barrier properties like aluminum foil (AL) or aluminum oxide (AlOx) coating. However, when selecting film materials, it's important to note that thicker doesn't always mean better. Thicker membranes of rigid materials might be more prone to folding and damage.
Medical Paper
Medical paper, also known as reinforced paper or dialysis paper is categorized based on weight per square meter. Common material specifications include 60 gsm (British paper), 70 gsm (domestic/Chinese paper), 80 gsm (French paper), which signify 60 g/m², 70 g/m², 80 g/m². When making a selection, the decision should be based on device risk assessment.
Unlike the high-density polyethylene of Tyvek®, medical paper is usually made from cellulose pulp, with lower puncture resistance and tear strength. It's susceptible to particle generation, and its fibers might turn yellow over time or when exposed to light—impacting aesthetics and leading to brittle fibers and packaging failure.
Medical paper is commonly used for Class I devices.
The final selection of medical device packaging should also consider material cost, environmental friendliness, and compliance with relevant regulations and standards. By choosing appropriate medical packaging materials, the safety, integrity, and effectiveness of medical supplies can be ensured, providing a better experience for both patients and healthcare professionals.



