One key function of medical packaging is to inform the end user about the packaged device and how to use it safely. Most of us know the iconic symbols found in medical packaging such as “sterile” or CE that effectively communicate to healthcare professionals about the device. Additionally, packaging design cues like chevron pouches and thumb notches help healthcare professionals to use proper opening techniques required for aseptic presentation. Things get more complicated with double or triple barrier packages when the validated sterile barrier is not clearly identifiable at the point-of-use. Thankfully, new sterile barrier symbols from the Sterile Barrier Association (SBA) will help to indicate just that!
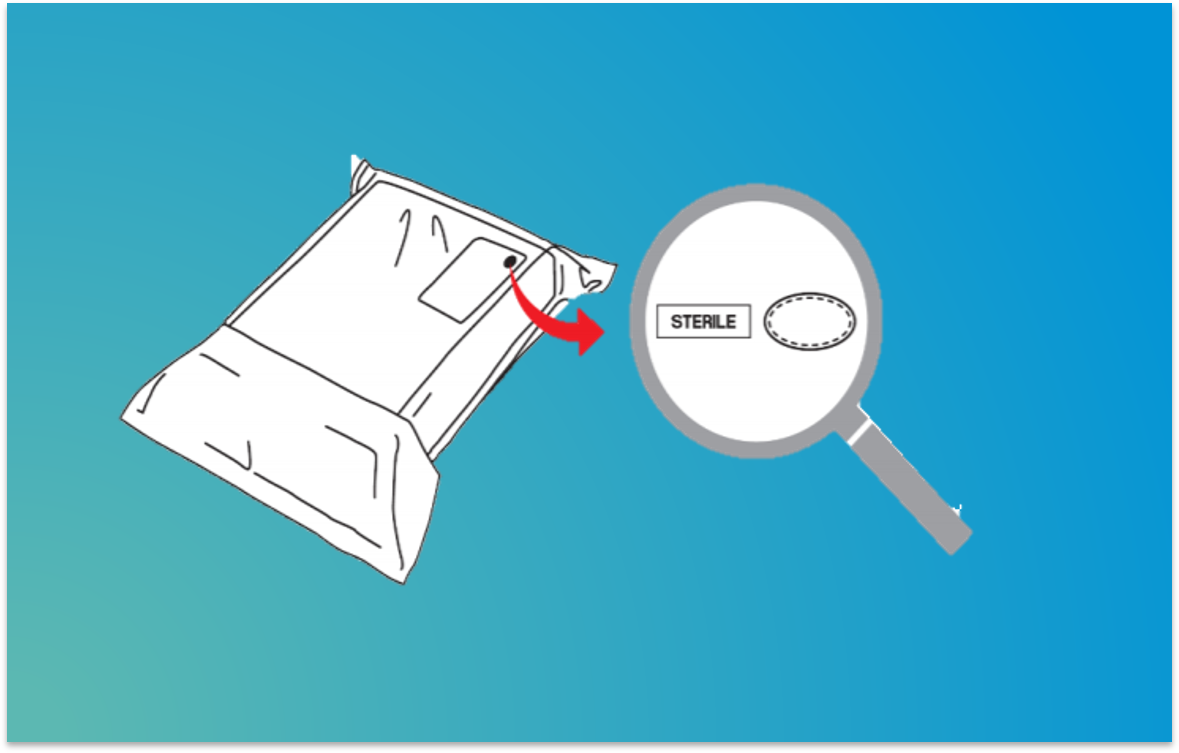
For example, implantable devices are often contained in multiple packaging layers consisting of protective packaging and the sterile barriers. After the easily distinguished, outermost protective packaging layers have been removed, we may find a double-entry pouch with two Tyvek® pouches containing the sterile medical device. Is the pouch inside the outer pouch sterile? Indeed, it would be impossible to differentiate a validated sterile barrier from protective packaging if the packaging doesn’t inform the end user about the difference.
Developing simple and intuitive symbols that are easy to remember and understand was a challenging task. Additional considerations require that the symbols represent all sterile packaging types and that they have a simple shape which is easy to print, even with a one-color, small resolution printer. After a significant effort, the new symbols were developed and then tested using an online survey with close to 500 entries from healthcare workers around the world. The test was deemed successful and thus the symbols were included in ISO 15223-1 – Medical Devices–Symbols to be used with medical device labels, labelling and information to be supplied.
The new symbols are composed from ovals which are formed either from a solid line (indicating a sterile barrier layer) or a dashed line (for a protective packaging layer). The oval should be placed next to or in combination with the sterile symbol. Understanding that available space in many medical devices is limited, placement of the sterile symbol within the oval provides a compact solution.
As with many regulations, it is left to companies to develop a solid rationale on the use of the new symbol, but having a robust packaging system design process in place helps significantly. Add in risk evaluation and companies should have a good rationale on whether the use of the symbol is needed. Above all, the purpose of the symbols is to make sterile packaging safe and effective for healthcare professionals and to eliminate the risk of infection of the patient.
For additional background and explanation, watch this brief video from Thierry Wagner, Director of Global Regulatory & Standards at DuPont™ Tyvek® Medical & Pharmaceutical Packaging.
PHOTO CREDIT: SBA