The Impact of Radiation Sterilization Modalities on Flexible Sterile Barrier Packaging
The health and safety of patients is the number one priority when it comes to sterile barrier packaging and sterilization. As a packaging engineer, it is important to understand the relationship between radiation sterilization modalities and flexible sterile barrier packaging materials, as well as the impact and dependency on each other.
When using radiation sterilization modalities, the level of absorbed dose is important to understand. Absorbed dose is the amount of energy that is deposited into a medium (in this case, the sterile barrier system, the protective packaging, and the packaged device). The more complex an organism is, the more susceptible to radiation it will be. More complex organisms will require less energy to be considered a lethal dose, while simple organisms like microbes will require more. The typical absorbed dose in an irradiation cycle can range up to 50 kGy. Comparing this to the lethal dose for a human being (5 Gy) shows the high amount of energy used in an irradiation sterilization cycle to ensure the sterility of a packaged device.
The three main modalities of radiation sterilization are: Gamma, E-beam, and X-ray.
Gamma has been in use for decades and is one of the most common sterilization modalities. Here you can see an animation that illustrates the gamma irradiation process. Multiple packaged products are loaded into a tote and indexed around a source rack containing Cobalt 60 pencils. The totes are exposed to photons as the Cobalt 60 decays, until the packaged product receives the full and specified dose.
E-beam is considered to be a high speed, high-capacity form of sterilization. It processes products extremely fast when compared to gamma (can be complete in 5-10 seconds depending on dose requirements) and provides less potential for oxidation and degradation of polymers. However, there are very specific packaging requirements for this type of sterilization due to its limited penetration. Due to this limitation, it is common for individual cases of products to be processed as opposed to large totes full of cases in gamma and X-ray. In this animation of the E-beam process, you can see individual cases are loaded directly onto a conveyor. The cases enter the radiation cell for exposure to a scan horn for the first exposure to E-beam irradiation. The cases are flipped and rotated between passes to ensure the appropriate amount of radiation is received.
X-ray is similar to Gamma, as the process of each produces photons which sterilize the packaged product. Compared to gamma, there is also a greater depth of penetration, therefore products with a higher density (ex: liquids or gels) are a good fit for this type of sterilization. The current state of X-ray sterilization has some inefficiencies since 87% of electrons are lost in the form of heat during the X-ray process. In this animation, you can see an example of an X-ray sterilization process. Like gamma, multiple packaged products are loaded into totes, then placed into a carrier and passed in front of the X-ray target. Like E-beam, the totes are then rotated 180 degrees and cycled past the X-ray target a second time. Finally, the totes are rotated from top to bottom and the cycle is repeated.
Comparing Gamma, E-beam, & X-ray
After breaking down each modality, let’s now look at a couple of the key similarities and differences between them.
-
Energy Transport: Gamma and X-ray delivers energy in the form of photons, while E-beam delivers energy in the form of electrons.
-
Delivery: Gamma’s beams are isotropic, while E-beam and X-ray are highly directional.
-
Reliability: Gamma is considered the most reliable.
-
Dose Rate: (The amount of time a product spends in front of the source to receive the full and specified dose.) Gamma takes hours, E-beam seconds, and X-ray minutes.
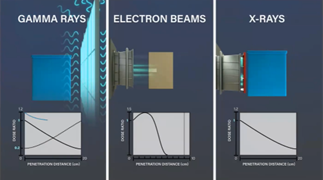
The animation below compares these processes by showing their effective depth of penetration and resulting dose profile. Gamma and E-Beam are very similar in the depth of penetration, the main difference being the source and the direction of the photons. With the E-beam process, the depth of penetration is much less with a single dose as the electrons have no charge or mass which causes scattering followed by a steep drop off in the graph as the electrons lose energy. The blue line in each graph shows the resulting delivered dose through the packaged product.
Compatibility with Sterilization Modalities
As a packaging engineer, it is most likely someone else that is identifying the sterilization modality to be used. Therefore, it is important to be involved early in the process so you can appropriately determine packaging materials that are compatible with the selected modality. AAMI TIR 17 is a key resource for understanding material compatibility, but it is also best practice to consult with your suppliers and existing test data to understand what is best before moving forward with your packaging design.
Most common packaging materials are compatible with these sterilization modalities. Some packaging design considerations:
-
Always monitor the effects on material attributes and seals after any type of sterilization and associated aging studies.
-
For Gamma, E-beam, & X-ray, Tyvek® is very commonly used. Since porosity is not required during irradiation, you can consider various types of flexible packaging configurations such as film/film and foil/foil based on your product needs.
-
Whenever you are not using a porous material, consider the effects of high-altitude shipments where pressure changes can occur. The air trapped inside of a package can try to escape and can lead to seal creep or sterile barrier breach.
-
Oliver Healthcare Packaging and Sterigenics performed a study to analyze the effects of Gamma, E-beam, & X-ray irradiation on typical flexible sterile barrier materials. The test results for coated Tyvek®, polyester and nylon-based films and foil structure were the same among all 3 modalities. Polypropylene and medical-based paper both had adverse effects from all 3 modalities, as explained in AAMI TIR 17.
Interested in learning more about radiation sterilization modalities and their impact on packaging? Check out this recent webinar I did with Chad Rhodes from Sterigenics.



