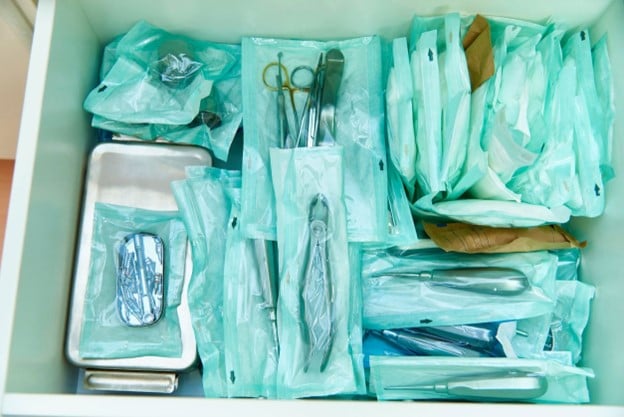
A sterile barrier system is often present (and mandated by regulation) in the medical packaging industry. The concept of sterilization—or rather, the need for it—has been around for a long time. In the mid 1800s, Louis Pasteur, who is recognized as one of the founding fathers of microbiology, identified that microorganisms were the root cause of food contamination. He introduced heating as a way to kill microorganisms. This same theory of sterilization has played out time and time again—from food to medicines to medical grade packaging.
An easy example of this is one we’re likely all familiar with, especially following the Covid-19 pandemic. Think about wiping down your kitchen counter and a clean cloth. The cloth would remove the surface dirt for sure. But if you use a disinfecting wipe or cleaner—something that says “kills 99.9% of viruses and bacteria”—you’ve now sterilized your counter as well.
Let’s now move on to sterile barrier systems, including what they are and why they’re so important in healthcare packaging. A sterile barrier system is defined as a part of ISO 11607. ISO 11607 specifies the requirements and test methods for the materials used in and the sterile barrier systems themselves, as well as preformed sterile barrier systems and packaging systems that are intended to maintain sterility until the point of use, which is usually a medical facility, operating room, or lab.
When discussing a sterile barrier system, we need to know more about the role it will play. By definition, a sterile barrier system minimizes the risk of ingress of microorganisms and allows for aseptic presentation of the sterile contents. When we talk about an SBS though, it is important to understand the difference between primary packaging and a more complete SBS. A sterile barrier system involves sterilization of the package in its entirety whereas a primary sterile barrier is the sterilized packaging that keeps a device or component safe. An SBS might be a surgical kit that includes multiple devices in a tray, with the tray inside a pouch. The full kit would be sterilized as it is, making it an SBS.
Now think about a kit that might not need to be fully sterilized. Maybe just a component or two in the kit must be sterile. Let’s put those in a small sterile pouch, then in the tray, then the larger pouch. In this instance, the primary sterile barrier would be the small sterile pouch.
Lastly, there is one other type of SBS defined in ISO 11607—the preformed sterile barrier system. A preformed SBS is a sterile barrier system that is supplied partially assembled for filling and final closure or sealing. An example of this is a pouch that is sealed on three sides, but then left open on the fourth side for filling and sealing.
At the end of the day, any kind of sterilized medical packaging is in place for one primary reason—to keep patients safe. It’s a big responsibility, which is why it is so heavily regulated … and packaging is just one part of the process.