For those in the medical device and healthcare packaging industries, whether you are just starting out or have been in the field for 30 years like me, we understand ISO 11607.
We live and breathe it every day. Whether we are considering the device itself, the package design, making a material selection, or considering sterilization and transportation, ISO 11607 is our guide.
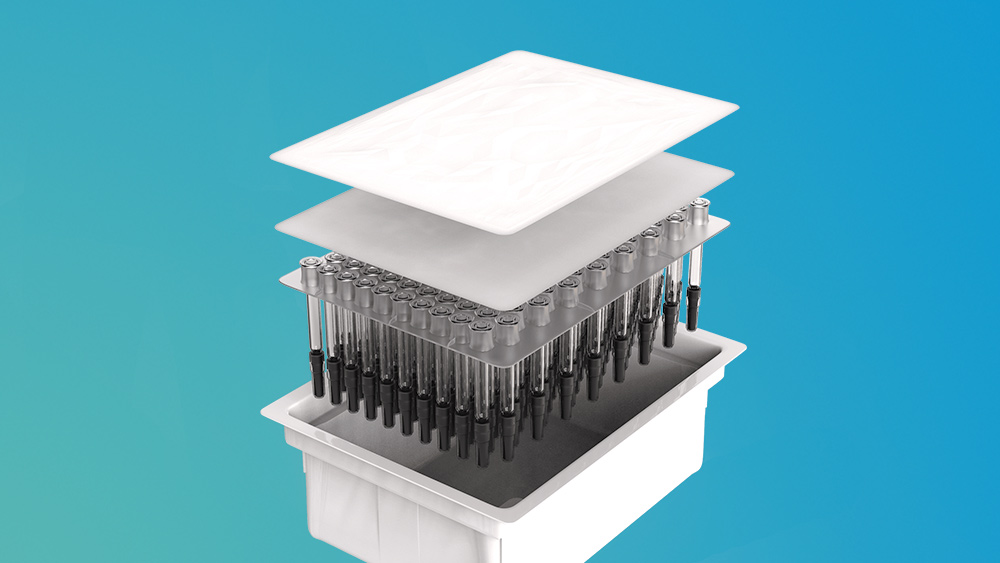
The Top 3 Things You Should Know about ISO 11040-7: Packaging In-Process Pharma and Prefilled Syringe Tubs
-
In the introduction, the standard states that the packaging design and material should be compatible with the process of the customer. So, what exactly does the process of the customer mean? In my experience, I have seen examples of manual processing and automated processing, as well as aseptic filling, peeling, and assembly by both humans and robotics. Comparing manual human processes to automated mechanical processes is perhaps the easiest comparison. Think about the package design alone, and what might need to be different in the two. The peel strength, location of the peel, whether heat is being applied prior to peeling, gripper size, package consistency … all of these would be impacted based on the process being used.
-
The terms decontamination and sterilization are used throughout the ISO document, and I have heard them used interchangeably in the industry. We must be cautious when using either as there are clear distinctions or implications for each. In the simplest terms, decontamination refers to everything exterior. For example, the outside of the syringe tub or the top of the tub lid. Sterilization, on the other hand, is used when discussing interior content, like the tub nest and the syringes themselves. Decontamination is usually done using vaporized hydrogen peroxide or VHP, while Ethylene Oxide is still the most popular sterilization modality for prefilled syringe tubs.
-
Section 4.1.3 is worth noting, as it offers processing guidance on each major component of a prefilled syringe tub, including: a. tubs, lids, and insert liners; b. nests; and c. protective bags. The steps under each component apply to both the manufacturer and the customer and can be used as a quick “cheat sheet” of the most common process steps.
Fun fact: In some ISO documents, when they refer to foil, as they do in section 3.2, foil can be an alternative term for film or substrate, as commonly used across Europe. An insert liner is rarely made of aluminum foil as we know it in the industry.
As a Technical Fellow at Oliver, and someone who has been deeply invested in our industry’s standards and regulations, it is my job to understand and interpret documentation like ISO 11040 and others. A good packaging partner should do that for you, so be sure to choose your partners wisely. It can only serve to make your process easier in the long run.
.png)